What Animal Uses The Proteites Of Density To Function
 | |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name H2o | |||
| Systematic IUPAC name Oxidane | |||
| Other names Hydrogen hydroxide (HH or HOH), hydrogen oxide, dihydrogen monoxide (DHMO) (systematic name[ane]), dihydrogen oxide, hydric acid, hydrohydroxic acrid, hydroxic acid, hydrol,[two] μ-oxido dihydrogen, κ1-hydroxyl hydrogen(0) | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| Beilstein Reference | 3587155 | ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| Gmelin Reference | 117 | ||
| PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemic formula | H 2 O | ||
| Molar mass | 18.01528(33) g/mol | ||
| Advent | White crystalline solid, almost colorless liquid with a hint of blue, colorless gas[3] | ||
| Odor | None | ||
| Density | Liquid:[four] 0.9998396 g/mL at 0 °C 0.9970474 one thousand/mL at 25 °C 0.961893 thousand/mL at 95 °C Solid:[v] 0.9167 g/ml at 0 °C | ||
| Melting point | 0.00 °C (32.00 °F; 273.15 K) [b] | ||
| Boiling point | 99.98 °C (211.96 °F; 373.13 K)[fifteen] [b] | ||
| Solubility in water | N/A | ||
| Solubility | Poorly soluble in haloalkanes, aliphatic and aromatic hydrocarbons, ethers.[half-dozen] Improved solubility in carboxylates, alcohols, ketones, amines. Miscible with methanol, ethanol, propanol, isopropanol, acetone, glycerol, 1,iv-dioxane, tetrahydrofuran, sulfolane, acetaldehyde, dimethylformamide, dimethoxyethane, dimethyl sulfoxide, acetonitrile. Partially miscible with diethyl ether, methyl ethyl ketone, dichloromethane, ethyl acetate, bromine. | ||
| Vapor force per unit area | 3.1690 kilopascals or 0.031276 atm at 25 °C[7] | ||
| Acidity (pThou a) | 13.995[8] [9] [a] | ||
| Basicity (pK b) | 13.995 | ||
| Cohabit acid | Hydronium H3O+ (pKa = 0) | ||
| Cohabit base of operations | Hydroxide OH– (pKb = 0) | ||
| Thermal conductivity | 0.6065 W/(grand·K)[12] | ||
| Refractive alphabetize (northward D) | i.3330 (20 °C)[xiii] | ||
| Viscosity | 0.890 mPa·s (0.890 cP)[14] | ||
| Structure | |||
| Crystal construction | Hexagonal | ||
| Bespeak group | C2v | ||
| Molecular shape | Bent | ||
| Dipole moment | 1.8546 D[16] | ||
| Thermochemistry | |||
| Heat capacity (C) | 75.385 ± 0.05 J/(mol·Thou)[17] | ||
| Std molar | 69.95 ± 0.03 J/(mol·Thousand)[17] | ||
| Std enthalpy of | −285.83 ± 0.04 kJ/mol[6] [17] | ||
| Gibbs gratis energy (Δf Chiliad˚) | −237.24 kJ/mol[6] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Master hazards | Drowning Avalanche (equally snow) | ||
| GHS labelling:[xviii] | |||
| Hazard statements | |||
| NFPA 704 (fire diamond) | 0 0 0 | ||
| Wink point | Non-flammable | ||
| Prophylactic data canvas (SDS) | SDS | ||
| Related compounds | |||
| Other cations | Hydrogen sulfide Hydrogen selenide Hydrogen telluride Hydrogen polonide Hydrogen peroxide | ||
| Related solvents | Acetone Methanol | ||
| Supplementary data page | |||
| H2o (information page) | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |||
Water (H
2 O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of bluish. It is by far the most studied chemic compound[19] and is described every bit the "universal solvent"[twenty] and the "solvent of life".[21] Information technology is the virtually abundant substance on the surface of Earth[22] and the merely common substance to exist as a solid, liquid, and gas on World's surface.[23] It is also the third most abundant molecule in the universe (behind molecular hydrogen and carbon monoxide).[22]
Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Its hydrogen bonding causes its many unique properties, such as having a solid form less dense than its liquid form,[c] a relatively loftier humid betoken of 100 °C for its molar mass, and a high heat capacity.
H2o is amphoteric, meaning that it can exhibit backdrop of an acid or a base of operations, depending on the pH of the solution that it is in; it readily produces both H +
and OH −
ions.[c] Related to its amphoteric grapheme, it undergoes self-ionization. The product of the activities, or approximately, the concentrations of H +
and OH −
is a abiding, then their respective concentrations are inversely proportional to each other.[24]
Physical backdrop [edit]
Water is the chemic substance with chemical formula H
2 O; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.[25] Water is a tasteless, odorless liquid at ambient temperature and pressure. Liquid water has weak assimilation bands at wavelengths of around 750 nm which cause it to appear to have a blue color.[3] This can hands be observed in a h2o-filled bathroom or wash-bowl whose lining is white. Large ice crystals, every bit in glaciers, likewise appear blue.
Under standard conditions, water is primarily a liquid, unlike other analogous hydrides of the oxygen family unit, which are more often than not gaseous. This unique belongings of water is due to hydrogen bonding. The molecules of water are constantly moving concerning each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds (two × 10−13 seconds).[26] However, these bonds are strong plenty to create many of the peculiar properties of h2o, some of which make it integral to life.
H2o, ice, and vapour [edit]
Inside the Earth's atmosphere and surface, the liquid stage is the virtually mutual and is the form that is generally denoted by the word "h2o". The solid stage of water is known as water ice and commonly takes the structure of hard, amalgamated crystals, such as water ice cubes, or loosely accumulated granular crystals, similar snowfall. Aside from mutual hexagonal crystalline water ice, other crystalline and amorphous phases of water ice are known. The gaseous stage of water is known equally water vapor (or steam). Visible steam and clouds are formed from minute aerosol of water suspended in the air.
Water also forms a supercritical fluid. The critical temperature is 647 K and the critical pressure is 22.064 MPa. In nature, this but rarely occurs in extremely hostile conditions. A likely example of naturally occurring supercritical water is in the hottest parts of deep h2o hydrothermal vents, in which water is heated to the critical temperature past volcanic plumes and the critical pressure level is caused past the weight of the ocean at the farthermost depths where the vents are located. This pressure is reached at a depth of about 2200 meters: much less than the mean depth of the ocean (3800 meters).[27]
Heat capacity and heats of vaporization and fusion [edit]

Rut of vaporization of water from melting to critical temperature
H2o has a very high specific heat chapters of 4184 J/(kg·M) at xx °C (4182 J/(kg·K) at 25 °C) —the 2nd-highest among all the heteroatomic species (subsequently ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling betoken), both of which are a effect of the extensive hydrogen bonding between its molecules. These ii unusual backdrop let water to moderate Earth'due south climate by buffering large fluctuations in temperature. Well-nigh of the additional energy stored in the climate system since 1970 has accumulated in the oceans.[28]
The specific enthalpy of fusion (more normally known every bit latent heat) of water is 333.55 kJ/kg at 0 °C: the aforementioned amount of energy is required to cook water ice as to warm ice from −160 °C up to its melting signal or to rut the same corporeality of water past most 80 °C. Of common substances, simply that of ammonia is higher. This holding confers resistance to melting on the ice of glaciers and migrate ice. Before and since the appearance of mechanical refrigeration, ice was and all the same is in common use for retarding nutrient spoilage.
The specific oestrus capacity of ice at −10 °C is 2030 J/(kg·K)[29] and the estrus capacity of steam at 100 °C is 2080 J/(kg·K).[30]
Density of h2o and ice [edit]

Density of ice and water as a part of temperature
The density of water is about one gram per cubic centimetre (62 lb/cu ft): this relationship was originally used to define the gram.[31] The density varies with temperature, but not linearly: as the temperature increases, the density rises to a height at iii.98 °C (39.xvi °F) and then decreases;[32] the initial increase is unusual because most liquids undergo thermal expansion so that the density only decreases as a role of temperature. The increase observed for water from 0 °C (32 °F) to 3.98 °C (39.xvi °F) and for a few other liquids[d] is described equally negative thermal expansion. Regular, hexagonal ice is likewise less dense than liquid water—upon freezing, the density of water decreases by about 9%.[35] [eastward]
These peculiar effects are due to the highly directional bonding of water molecules via the hydrogen bonds: ice and liquid h2o at depression temperature take comparatively low-density, low-energy open lattice structures. The breaking of hydrogen bonds on melting with increasing temperature in the range 0–4 °C allows for a denser molecular packing in which some of the lattice cavities are filled by h2o molecules.[32] [36] Above 4 °C, even so, thermal expansion becomes the dominant upshot,[36] and water near the boiling point (100 °C) is near 4% less dense than water at iv °C (39 °F).[35] [f]
Nether increasing pressure, ice undergoes a number of transitions to other polymorphs with higher density than liquid water, such as ice II, ice III, high-density amorphous water ice (HDA), and very-high-density amorphous ice (VHDA).[37] [38]
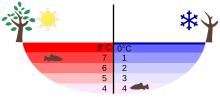
Temperature distribution in a lake in summer and winter
The unusual density curve and lower density of ice than of water is essential for much of the life on earth—if water were most dumbo at the freezing betoken, then in winter the cooling at the surface would lead to convective mixing. Once 0 °C are reached, the water body would freeze from the bottom up, and all life in it would be killed.[35] Furthermore, given that water is a good thermal insulator (due to its oestrus capacity), some frozen lakes might not completely thaw in summertime.[35] As it is, the inversion of the density curve leads to a stable layering for surface temperatures below iv °C, and with the layer of water ice that floats on top insulating the water below,[39] even e.thousand., Lake Baikal in central Siberia freezes only to about 1 thousand thickness in wintertime. In general, for deep plenty lakes, the temperature at the lesser stays constant at about 4 °C (39 °F) throughout the yr (see diagram).[35]
Density of saltwater and ice [edit]

The density of saltwater depends on the dissolved table salt content equally well as the temperature. Ice still floats in the oceans, otherwise, they would freeze from the bottom up. Nonetheless, the common salt content of oceans lowers the freezing point by nigh 1.nine °C[40] (see here for explanation) and lowers the temperature of the density maximum of water to the one-time freezing point at 0 °C. This is why, in ocean water, the downward convection of colder water is not blocked past an expansion of water as it becomes colder nearly the freezing point. The oceans' cold h2o well-nigh the freezing point continues to sink. So creatures that live at the bottom of cold oceans like the Chill Sea generally alive in water 4 °C colder than at the bottom of frozen-over fresh water lakes and rivers.
Every bit the surface of saltwater begins to freeze (at −one.nine °C[40] for normal salinity seawater, 3.v%) the ice that forms is essentially table salt-free, with about the same density as freshwater ice. This ice floats on the surface, and the salt that is "frozen out" adds to the salinity and density of the seawater just below it, in a process known as alkali rejection. This denser saltwater sinks by convection and the replacing seawater is subject to the aforementioned process. This produces substantially freshwater ice at −1.ix °C[40] on the surface. The increased density of the seawater beneath the forming ice causes information technology to sink towards the lesser. On a large calibration, the process of alkali rejection and sinking common cold salty h2o results in body of water currents forming to transport such water away from the Poles, leading to a global arrangement of currents chosen the thermohaline circulation.
Miscibility and condensation [edit]

Cherry line shows saturation
Water is miscible with many liquids, including ethanol in all proportions. Water and well-nigh oils are immiscible usually forming layers according to increasing density from the height. This tin can be predicted by comparing the polarity. Water beingness a relatively polar compound will tend to be miscible with liquids of high polarity such as ethanol and acetone, whereas compounds with low polarity will tend to be immiscible and poorly soluble such as with hydrocarbons.
Equally a gas, water vapor is completely miscible with air. On the other paw, the maximum water vapor force per unit area that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For instance, if the vapor's fractional pressure level is 2% of atmospheric force per unit area and the air is cooled from 25 °C, starting at well-nigh 22 °C, water will start to condense, defining the dew point, and creating fog or dew. The reverse process accounts for the fog burning off in the morning. If the humidity is increased at room temperature, for example, by running a hot shower or a bath, and the temperature stays about the same, the vapor shortly reaches the pressure for stage modify and then condenses out as minute h2o droplets, unremarkably referred to every bit steam.
A saturated gas or 1 with 100% relative humidity is when the vapor pressure of water in the air is at equilibrium with vapor pressure level due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in the air is small-scale, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful. Vapor pressure above 100% relative humidity is called supersaturated and tin can occur if the air is rapidly cooled, for example, by rising all of a sudden in an updraft.[g]
Vapor force per unit area [edit]
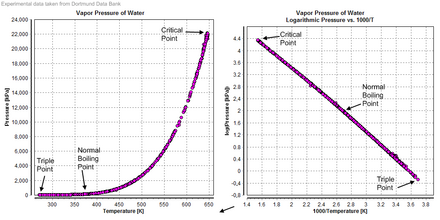
Vapor force per unit area diagrams of water
Compressibility [edit]
The compressibility of water is a role of pressure and temperature. At 0 °C, at the limit of nix pressure, the compressibility is 5.one×10−10 Pa−1 . At the cipher-pressure limit, the compressibility reaches a minimum of 4.4×ten−10 Pa−1 effectually 45 °C before increasing again with increasing temperature. Every bit the pressure is increased, the compressibility decreases, being three.9×ten−x Pa−1 at 0 °C and 100 megapascals (i,000 bar).[41]
The bulk modulus of water is about 2.ii GPa.[42] The low compressibility of not-gasses, and of h2o in particular, leads to their often being assumed as incompressible. The depression compressibility of water means that fifty-fifty in the deep oceans at iv km depth, where pressures are 40 MPa, there is only a 1.8% decrease in volume.[42]
The majority modulus of water water ice ranges from eleven.iii GPa at 0 K upward to 8.6 GPa at 273 K.[43] The large change in the compressibility of ice every bit a function of temperature is the consequence of its relatively large thermal expansion coefficient compared to other common solids.
Triple indicate [edit]

The solid/liquid/vapour triple point of liquid water, water ice Ih and water vapor in the lower left portion of a water phase diagram.
The temperature and pressure level at which ordinary solid, liquid, and gaseous water coexist in equilibrium is a triple indicate of water. Since 1954, this point had been used to define the base unit of temperature, the kelvin,[44] [45] but, starting in 2019, the kelvin is now defined using the Boltzmann constant, rather than the triple point of water.[46]
Due to the existence of many polymorphs (forms) of ice, water has other triple points, which have either three polymorphs of ice or ii polymorphs of ice and liquid in equilibrium.[45] Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented farther triple points in the 1960s.[47] [48] [49]
| Phases in stable equilibrium | Pressure | Temperature |
|---|---|---|
| liquid water, ice Ih, and water vapor | 611.657 Pa[50] | 273.16 K (0.01 °C) |
| liquid water, ice Ih, and ice Three | 209.nine MPa | 251 Chiliad (−22 °C) |
| liquid water, ice 3, and water ice V | 350.1 MPa | −17.0 °C |
| liquid h2o, ice V, and ice VI | 632.4 MPa | 0.16 °C |
| ice Ih, Water ice II, and ice Three | 213 MPa | −35 °C |
| water ice Ii, ice III, and ice V | 344 MPa | −24 °C |
| ice Ii, ice V, and ice 6 | 626 MPa | −70 °C |
Melting point [edit]
The melting indicate of ice is 0 °C (32 °F; 273 K) at standard pressure; nonetheless, pure liquid water tin can be supercooled well below that temperature without freezing if the liquid is non mechanically disturbed. Information technology can remain in a fluid state downwardly to its homogeneous nucleation point of about 231 K (−42 °C; −44 °F).[51] The melting indicate of ordinary hexagonal ice falls slightly nether moderately high pressures, past 0.0073 °C (0.0131 °F)/atm[h] or virtually 0.five °C (0.xc °F)/70 atm[i] [52] as the stabilization energy of hydrogen bonding is exceeded by intermolecular repulsion, but equally ice transforms into its polymorphs (see crystalline states of water ice) above 209.9 MPa (ii,072 atm), the melting betoken increases markedly with pressure, i.e., reaching 355 Chiliad (82 °C) at 2.216 GPa (21,870 atm) (triple indicate of Ice 7[53]).
Electrical backdrop [edit]
Electrical conductivity [edit]
Pure water containing no exogenous ions is an excellent electronic insulator, but not even "deionized" water is completely free of ions. H2o undergoes autoionization in the liquid country when two h2o molecules form ane hydroxide anion (OH −
) and ane hydronium cation (H
iii O +
). Because of autoionization, at ambience temperatures pure liquid water has a similar intrinsic charge carrier concentration to the semiconductor germanium and an intrinsic accuse carrier concentration three orders of magnitude greater than the semiconductor silicon, hence, based on accuse carrier concentration, water can not be considered to be a completely dielectric material or electrical insulator but to be a express conductor of ionic charge.[54]
Because water is such a good solvent, it nearly ever has some solute dissolved in it, ofttimes a salt. If water has fifty-fifty a tiny amount of such an impurity, then the ions can carry charges back and forth, assuasive the water to carry electricity far more readily.
It is known that the theoretical maximum electrical resistivity for water is approximately eighteen.2 MΩ·cm (182 kΩ·m) at 25 °C.[55] This figure agrees well with what is typically seen on reverse osmosis, ultra-filtered and deionized ultra-pure water systems used, for instance, in semiconductor manufacturing plants. A salt or acrid contaminant level exceeding even 100 parts per trillion (ppt) in otherwise ultra-pure water begins to noticeably lower its resistivity by up to several kΩ·m.[ citation needed ]
In pure h2o, sensitive equipment can observe a very slight electrical electrical conductivity of 0.05501 ± 0.0001 μS/cm at 25.00 °C.[55] Water can also be electrolyzed into oxygen and hydrogen gases but in the absence of dissolved ions this is a very ho-hum process, as very trivial current is conducted. In ice, the chief charge carriers are protons (run into proton conductor).[56] Water ice was previously thought to have a small merely measurable conductivity of one×ten −10 South/cm, merely this conductivity is now thought to be nearly entirely from surface defects, and without those, water ice is an insulator with an immeasurably small-scale conductivity.[32]
Polarity and hydrogen bonding [edit]

A diagram showing the partial charges on the atoms in a water molecule
An important feature of water is its polar nature. The structure has a bent molecular geometry for the two hydrogens from the oxygen vertex. The oxygen atom too has two solitary pairs of electrons. One effect ordinarily ascribed to the lone pairs is that the H–O–H gas-phase bend bending is 104.48°,[57] which is smaller than the typical tetrahedral bending of 109.47°. The lone pairs are closer to the oxygen atom than the electrons sigma bonded to the hydrogens, and so they crave more space. The increased repulsion of the lone pairs forces the O–H bonds closer to each other. [58]
Another result of its construction is that water is a polar molecule. Due to the departure in electronegativity, a bond dipole moment points from each H to the O, making the oxygen partially negative and each hydrogen partially positive. A large molecular dipole, points from a region between the two hydrogen atoms to the oxygen atom. The charge differences cause water molecules to aggregate (the relatively positive areas being attracted to the relatively negative areas). This attraction, hydrogen bonding, explains many of the backdrop of water, such as its solvent properties.[59]
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for several of the water's concrete properties. These properties include its relatively high melting and boiling point temperatures: more free energy is required to pause the hydrogen bonds betwixt water molecules. In contrast, hydrogen sulfide (H
2 S), has much weaker hydrogen bonding due to sulfur'due south lower electronegativity. H
2 S is a gas at room temperature, despite hydrogen sulfide having most twice the tooth mass of water. The extra bonding between water molecules likewise gives liquid water a large specific rut chapters. This loftier estrus capacity makes water a proficient heat storage medium (coolant) and estrus shield.
Cohesion and adhesion [edit]

Water molecules stay close to each other (cohesion), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds beingness formed with different h2o molecules; merely at any given time in a sample of liquid h2o, a big portion of the molecules are held together by such bonds.[sixty]
Water also has high adhesion properties because of its polar nature. On clean, smooth glass the water may form a thin picture show considering the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces.[ citation needed ] In biological cells and organelles, water is in contact with membrane and protein surfaces that are hydrophilic; that is, surfaces that have a potent attraction to water. Irving Langmuir observed a strong repulsive force betwixt hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very big but subtract rapidly over a nanometer or less.[61] They are important in biology, particularly when cells are dehydrated past exposure to dry out atmospheres or to extracellular freezing.[62]

Surface tension [edit]

This newspaper clip is nether the water level, which has risen gently and smoothly. Surface tension prevents the clip from submerging and the water from alluvion the glass edges.

Temperature dependence of the surface tension of pure water
H2o has an unusually loftier surface tension of 71.99 mN/m at 25 °C[63] which is caused by the strength of the hydrogen bonding between water molecules.[64] This allows insects to walk on water.[64]
Capillary action [edit]
Because water has potent cohesive and adhesive forces, it exhibits capillary action.[65] Strong cohesion from hydrogen bonding and adhesion allows trees to transport water more than than 100 one thousand upwardly.[64]
Water equally a solvent [edit]

Water is an splendid solvent due to its high dielectric abiding.[66] Substances that mix well and deliquesce in h2o are known as hydrophilic ("water-loving") substances, while those that practice not mix well with water are known as hydrophobic ("h2o-fearing") substances.[67] The ability of a substance to deliquesce in water is determined by whether or not the substance can lucifer or better the strong attractive forces that water molecules generate betwixt other water molecules. If a substance has properties that practise non allow information technology to overcome these stiff intermolecular forces, the molecules are precipitated out from the h2o. Contrary to the common misconception, h2o and hydrophobic substances practise non "repel", and the hydration of a hydrophobic surface is energetically, but non entropically, favorable.
When an ionic or polar compound enters water, information technology is surrounded by water molecules (hydration). The relatively pocket-sized size of water molecules (~ 3 angstroms) allows many water molecules to environment ane molecule of solute. The partially negative dipole ends of the h2o are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In full general, ionic and polar substances such as acids, alcohols, and salts are relatively soluble in water, and nonpolar substances such as fats and oils are non. Nonpolar molecules stay together in water because it is energetically more than favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions with non-polar molecules.
An example of an ionic solute is tabular array salt; the sodium chloride, NaCl, separates into Na +
cations and Cl −
anions, each existence surrounded past water molecules. The ions are and then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and permit it to be carried abroad into solution.
Breakthrough tunneling [edit]
The quantum tunneling dynamics in water was reported equally early every bit 1992. At that time it was known that there are motions which destroy and regenerate the weak hydrogen bond by internal rotations of the substituent water monomers.[68] On xviii March 2016, it was reported that the hydrogen bail can be cleaved by quantum tunneling in the water hexamer. Unlike previously reported tunneling motions in h2o, this involved the concerted breaking of two hydrogen bonds.[69] Later in the same year, the discovery of the quantum tunneling of water molecules was reported.[70]
Electromagnetic absorption [edit]
Water is relatively transparent to visible light, near ultraviolet light, and far-ruby calorie-free, but it absorbs most ultraviolet light, infrared light, and microwaves. Most photoreceptors and photosynthetic pigments utilize the portion of the light spectrum that is transmitted well through water. Microwave ovens take advantage of water'southward opacity to microwave radiations to heat the water inside of foods. Water's light bluish colour is caused past weak absorption in the red part of the visible spectrum.[three] [71]
Structure [edit]

A single water molecule tin participate in a maximum of four hydrogen bonds because information technology can accept ii bonds using the lone pairs on oxygen and donate 2 hydrogen atoms. Other molecules like hydrogen fluoride, ammonia, and methanol tin likewise form hydrogen bonds. However, they practise non evidence anomalous thermodynamic, kinetic, or structural backdrop like those observed in water because none of them can course four hydrogen bonds: either they cannot donate or have hydrogen atoms, or there are steric effects in bulky residues. In water, intermolecular tetrahedral structures form due to the 4 hydrogen bonds, thereby forming an open up structure and a three-dimensional bonding network, resulting in the dissonant decrease in density when cooled below iv °C. This repeated, constantly reorganizing unit defines a three-dimensional network extending throughout the liquid. This view is based upon neutron scattering studies and calculator simulations, and it makes sense in the light of the unambiguously tetrahedral arrangement of water molecules in water ice structures.
All the same, there is an alternative theory for the construction of h2o. In 2004, a controversial paper from Stockholm University suggested that h2o molecules in the liquid state typically demark not to iv but only two others; thus forming chains and rings. The term "string theory of water" (which is not to be confused with the string theory of physics) was coined. These observations were based upon X-ray assimilation spectroscopy that probed the local environs of individual oxygen atoms.[72]
Molecular construction [edit]
The repulsive effects of the 2 lone pairs on the oxygen atom cause water to have a bent, not linear, molecular construction,[73] allowing information technology to be polar. The hydrogen–oxygen–hydrogen angle is 104.45°, which is less than the 109.47° for platonic spthree hybridization. The valence bond theory caption is that the oxygen atom's solitary pairs are physically larger and therefore take upward more than space than the oxygen cantlet's bonds to the hydrogen atoms.[74] The molecular orbital theory explanation (Bent's rule) is that lowering the energy of the oxygen atom's nonbonding hybrid orbitals (by assigning them more than s character and less p character) and correspondingly raising the free energy of the oxygen atom's hybrid orbitals bonded to the hydrogen atoms (by assigning them more p character and less southward character) has the net effect of lowering the energy of the occupied molecular orbitals considering the energy of the oxygen atom'south nonbonding hybrid orbitals contributes completely to the free energy of the oxygen atom'due south lone pairs while the free energy of the oxygen atom's other two hybrid orbitals contributes only partially to the energy of the bonding orbitals (the residual of the contribution coming from the hydrogen atoms' 1s orbitals).
Chemic properties [edit]
Self-ionization [edit]
In liquid water there is some self-ionization giving hydronium ions and hydroxide ions.
- two H
ii O ⇌ H
iii O +
+ OH −
The equilibrium constant for this reaction, known equally the ionic production of h2o, , has a value of about 10 −fourteen at 25 °C. At neutral pH, the concentration of the hydroxide ion (OH −
) equals that of the (solvated) hydrogen ion (H +
), with a value close to 10−7 mol Fifty−i at 25 °C.[75] See information folio for values at other temperatures.
The thermodynamic equilibrium constant is a quotient of thermodynamic activities of all products and reactants including water:
However for dilute solutions, the activity of a solute such equally H3O+ or OH− is approximated by its concentration, and the action of the solvent HtwoO is approximated by i, and then that we obtain the simple ionic production
Geochemistry [edit]
The action of water on stone over long periods of time typically leads to weathering and h2o erosion, physical processes that convert solid rocks and minerals into soil and sediment, just nether some conditions chemical reactions with water occur every bit well, resulting in metasomatism or mineral hydration, a type of chemical alteration of a rock which produces clay minerals. Information technology also occurs when Portland cement hardens.
Water water ice can form clathrate compounds, known as clathrate hydrates, with a variety of small molecules that can exist embedded in its spacious crystal lattice. The well-nigh notable of these is methane clathrate, 4 CH
4 ·23H
2 O, naturally constitute in large quantities on the ocean floor.
Acidity in nature [edit]
Pelting is generally mildly acidic, with a pH betwixt v.2 and five.8 if non having any acid stronger than carbon dioxide.[76] If high amounts of nitrogen and sulfur oxides are present in the air, they too will dissolve into the cloud and raindrops, producing acrid rain.
Isotopologues [edit]
Several isotopes of both hydrogen and oxygen be, giving rise to several known isotopologues of h2o. Vienna Standard Mean Ocean Water is the current international standard for water isotopes. Naturally occurring water is almost completely composed of the neutron-less hydrogen isotope protium. Only 155 ppm include deuterium ( two
H or D), a hydrogen isotope with 1 neutron, and fewer than twenty parts per quintillion include tritium ( 3
H or T), which has two neutrons. Oxygen also has iii stable isotopes, with 16
O nowadays in 99.76%, 17
O in 0.04%, and 18
O in 0.2% of water molecules.[77]
Deuterium oxide, D
2 O, is also known equally heavy water because of its higher density. It is used in nuclear reactors as a neutron moderator. Tritium is radioactive, decaying with a half-life of 4500 days; THO exists in nature only in infinitesimal quantities, being produced primarily via catholic ray-induced nuclear reactions in the atmosphere. Water with one protium and one deuterium atom HDO occur naturally in ordinary h2o in low concentrations (~0.03%) and D
two O in far lower amounts (0.000003%) and any such molecules are temporary every bit the atoms recombine.
The most notable physical differences between H
2 O and D
2 O, other than the uncomplicated divergence in specific mass, involve properties that are affected by hydrogen bonding, such as freezing and boiling, and other kinetic effects. This is because the nucleus of deuterium is twice as heavy as protium, and this causes noticeable differences in bonding energies. The difference in boiling points allows the isotopologues to exist separated. The self-diffusion coefficient of H
2 O at 25 °C is 23% college than the value of D
2 O.[78] Because water molecules exchange hydrogen atoms with one another, hydrogen deuterium oxide (DOH) is much more mutual in low-purity heavy water than pure dideuterium monoxide D
2 O.
Consumption of pure isolated D
ii O may affect biochemical processes—ingestion of large amounts impairs kidney and primal nervous system office. Small quantities can exist consumed without whatever ill-effects; humans are generally unaware of taste differences,[79] simply sometimes report a called-for sensation[80] or sweet season.[81] Very large amounts of heavy water must be consumed for any toxicity to go apparent. Rats, all the same, are able to avoid heavy water by aroma, and it is toxic to many animals.[82]
Low-cal water refers to deuterium-depleted water (DDW), water in which the deuterium content has been reduced below the standard 155 ppm level.
Occurrence [edit]
Water is the virtually abundant substance on Earth and as well the third most abundant molecule in the universe, afterward H
2 and CO.[22] 0.23 ppm of the earth's mass is water and 97.39% of the global water volume of 1.38×ten 9 kmiii is found in the oceans.[83]
Reactions [edit]
Acrid-base of operations reactions [edit]
Water is amphoteric: it has the ability to human action as either an acrid or a base in chemic reactions.[84] According to the Brønsted-Lowry definition, an acid is a proton (H +
) donor and a base is a proton acceptor.[85] When reacting with a stronger acid, water acts every bit a base; when reacting with a stronger base, information technology acts as an acrid.[85] For case, water receives an H +
ion from HCl when muriatic acid is formed:
- HCl
(acid) + H
two O
(base) ⇌ H
iii O +
+ Cl −
In the reaction with ammonia, NH
3 , water donates a H +
ion, and is thus acting equally an acid:
- NH
3
(base) + H
2 O
(acrid) ⇌ NH +
iv + OH −
Because the oxygen atom in water has two lone pairs, water oft acts as a Lewis base of operations, or electron-pair donor, in reactions with Lewis acids, although it tin can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water. HSAB theory describes water as both a weak hard acrid and a weak hard base of operations, meaning that it reacts preferentially with other difficult species:
- H +
(Lewis acid) + H
2 O
(Lewis base) → H
three O +
- Fe iii+
(Lewis acid) + H
ii O
(Lewis base) → Atomic number 26(H
2 O) three+
half dozen
- Cl −
(Lewis base) + H
2 O
(Lewis acid) → Cl(H
ii O) −
6
When a table salt of a weak acid or of a weak base is dissolved in water, water can partially hydrolyze the table salt, producing the corresponding base or acid, which gives aqueous solutions of soap and blistering soda their basic pH:
- Na
2 CO
three + H
2 O ⇌ NaOH + NaHCO
3
Ligand chemistry [edit]
Water's Lewis base graphic symbol makes it a common ligand in transition metal complexes, examples of which include metallic aquo complexes such as Fe(H
2 O) ii+
6 to perrhenic acid, which contains two h2o molecules coordinated to a rhenium center. In solid hydrates, h2o tin be either a ligand or simply lodged in the framework, or both. Thus, FeSO
4 ·7H
2 O consists of [Atomic number 262(HiiO)6]two+ centers and one "lattice h2o". H2o is typically a monodentate ligand, i.e., information technology forms only one bond with the fundamental cantlet.[86]

Some hydrogen-bonding contacts in FeSO4 .7HtwoO. This metallic aquo complex crystallizes with ane molecule of "lattice" water, which interacts with the sulfate and with the [Atomic number 26(H2O)6]two+ centers.
Organic chemical science [edit]
As a hard base, water reacts readily with organic carbocations; for example in a hydration reaction, a hydroxyl group (OH −
) and an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond, resulting in an alcohol. When the addition of water to an organic molecule cleaves the molecule in 2, hydrolysis is said to occur. Notable examples of hydrolysis are the saponification of fats and the digestion of proteins and polysaccharides. Water can also be a leaving grouping in SN2 exchange and E2 elimination reactions; the latter is and so known as a dehydration reaction.
Water in redox reactions [edit]
Water contains hydrogen in the oxidation state +1 and oxygen in the oxidation state −two.[87] It oxidizes chemicals such every bit hydrides, alkali metals, and some element of group i earth metals.[88] [89] One example of an alkali metallic reacting with water is:[xc]
- 2 Na + 2 H
2 O → H
2 + 2 Na +
+ two OH −
Some other reactive metals, such equally aluminum and beryllium, are oxidized by water as well, simply their oxides attach to the metal and form a passive protective layer.[91] Note that the rusting of iron is a reaction between iron and oxygen[92] that is dissolved in water, non between iron and water.
Water can be oxidized to emit oxygen gas, but very few oxidants react with h2o fifty-fifty if their reduction potential is greater than the potential of O
2 /H
2 O. Well-nigh all such reactions require a catalyst.[93] An case of the oxidation of h2o is:
- 4 AgF
ii + 2 H
2 O → four AgF + 4 HF + O
2
Electrolysis [edit]
Water can exist split into its elective elements, hydrogen, and oxygen, by passing an electric electric current through it.[94] This process is called electrolysis. The cathode half reaction is:
- ii H +
+ 2
e −
→ H
2
The anode one-half reaction is:
- 2 H
2 O → O
2 + 4 H +
+ four
east −
The gases produced bubble to the surface, where they tin be collected or ignited with a flame above the water if this was the intention. The required potential for the electrolysis of pure water is 1.23 V at 25 °C.[94] The operating potential is actually 1.48 Five or college in practical electrolysis.
History [edit]
Henry Cavendish showed that h2o was equanimous of oxygen and hydrogen in 1781.[95] The first decomposition of water into hydrogen and oxygen, past electrolysis, was done in 1800 by English chemist William Nicholson and Anthony Carlisle.[95] [96] In 1805, Joseph Louis Gay-Lussac and Alexander von Humboldt showed that water is composed of two parts hydrogen and one part oxygen.[97]
Gilbert Newton Lewis isolated the first sample of pure heavy h2o in 1933.[98]
The backdrop of h2o have historically been used to define diverse temperature scales. Notably, the Kelvin, Celsius, Rankine, and Fahrenheit scales were, or currently are, defined past the freezing and humid points of h2o. The less common scales of Delisle, Newton, Réaumur, and Rømer were defined similarly. The triple signal of water is a more commonly used standard point today.
Nomenclature [edit]
The accustomed IUPAC name of water is oxidane or simply water,[99] or its equivalent in dissimilar languages, although in that location are other systematic names which can be used to describe the molecule. Oxidane is only intended to exist used every bit the proper noun of the mononuclear parent hydride used for naming derivatives of h2o by substituent nomenclature.[100] These derivatives unremarkably have other recommended names. For example, the name hydroxyl is recommended over oxidanyl for the –OH group. The name oxane is explicitly mentioned by the IUPAC equally being unsuitable for this purpose, since information technology is already the name of a circadian ether also known as tetrahydropyran.[101] [102]
The simplest systematic proper noun of water is hydrogen oxide. This is analogous to related compounds such as hydrogen peroxide, hydrogen sulfide, and deuterium oxide (heavy water). Using chemical nomenclature for type I ionic binary compounds, water would take the name hydrogen monoxide,[103] but this is not among the names published by the International Matrimony of Pure and Applied Chemistry (IUPAC).[99] Another proper noun is dihydrogen monoxide, which is a rarely used proper name of water, and by and large used in the dihydrogen monoxide parody.
Other systematic names for water include hydroxic acid, hydroxylic acid, and hydrogen hydroxide, using acrid and base of operations names.[j] None of these exotic names are used widely. The polarized form of the water molecule, H +
OH −
, is likewise called hydron hydroxide by IUPAC nomenclature.[104]
H2o substance is a term used for hydrogen oxide (H2O) when one does non wish to specify whether 1 is speaking of liquid water, steam, some class of water ice, or a component in a mixture or mineral.
See as well [edit]
- Chemical bonding of water
- Dihydrogen monoxide parody
- Double distilled water
- Electromagnetic assimilation by water
- Fluid dynamics
- Hard water
- Heavy water
- Hydrogen polyoxide
- Ice
- Optical properties of water and ice
- Steam
- Superheated water
- Viscosity § Water
- Water cluster
- H2o (information page)
- H2o dimer
- H2o model
- Water thread experiment
Footnotes [edit]
- ^ A ordinarily quoted value of xv.7 used mainly in organic chemistry for the pKa of h2o is incorrect.[ten] [11]
- ^ a b Vienna Standard Hateful Ocean Water (VSMOW), used for calibration, melts at 273.1500089(10) Thousand (0.000089(10) °C, and boils at 373.1339 K (99.9839 °C). Other isotopic compositions melt or boil at slightly different temperatures.
- ^ a b H+ represents H
3 O +
(H
2 O)
northward and more circuitous ions that form. - ^ Negative thermal expansion is too observed in molten silica.[33] Too, fairly pure silicon has a negative coefficient of thermal expansion for temperatures between about xviii and 120 kelvins.[34]
- ^ Other substances that expand on freezing are silicon (melting point of ane,687 K (1,414 °C; two,577 °F)), gallium (melting point of 303 Thou (30 °C; 86 °F), germanium (melting point of one,211 Thousand (938 °C; 1,720 °F)), and bismuth (melting point of 545 K (272 °C; 521 °F))
- ^ (one-0.95865/1.00000) × 100% = 4.135%
- ^ Adiabatic cooling resulting from the ideal gas law.
- ^ The source gives it as 0.0072°C/atm. However the writer defines an temper equally 1,000,000 dynes/cmtwo (a bar). Using the standard definition of temper, ane,013,250 dynes/cmii, it works out to 0.0073°C/atm.
- ^ Using the fact that 0.5/0.0073 = 68.5.
- ^ Both acrid and base names exist for h2o because it is amphoteric (able to react both as an acid or an alkali).
References [edit]
Notes [edit]
- ^ "naming molecular compounds". www.iun.edu. Archived from the original on 24 September 2018. Retrieved 1 October 2018.
Sometimes these compounds have generic or common names (eastward.k., H2O is "water") and they also take systematic names (e.yard., H2O, dihydrogen monoxide).
- ^ "Definition of Hydrol". Merriam-Webster . Retrieved 21 April 2019.
- ^ a b c Braun, Charles L.; Smirnov, Sergei N. (1993-08-01). "Why is water blue?" (PDF). Periodical of Chemic Instruction. 70 (8): 612. Bibcode:1993JChEd..70..612B. doi:10.1021/ed070p612. ISSN 0021-9584.
- ^ Riddick 1970, Table of Physical Properties, H2o 0b. pg 67-viii.
- ^ Lide 2003, Backdrop of Ice and Supercooled Water in Department 6.
- ^ a b c Anatolievich, Kiper Ruslan. "Properties of substance: water".
- ^ Lide 2003, Vapor Pressure of Water From 0 to 370° C in Sec. 6.
- ^ Lide 2003, Chapter 8: Dissociation Constants of Inorganic Acids and Bases.
- ^ Weingärtner et al. 2016, p. 13.
- ^ "What is the pKa of Water". University of California, Davis. 2015-08-09.
- ^ Silverstein, Todd P.; Heller, Stephen T. (17 April 2017). "pKa Values in the Undergraduate Curriculum: What Is the Existent pKa of H2o?". Journal of Chemical Education. 94 (six): 690–695. Bibcode:2017JChEd..94..690S. doi:ten.1021/acs.jchemed.6b00623.
- ^ Ramires, Maria L. V.; Castro, Carlos A. Nieto de; Nagasaka, Yuchi; Nagashima, Akira; Assael, Marc J.; Wakeham, William A. (1995-05-01). "Standard Reference Data for the Thermal Electrical conductivity of H2o". Journal of Physical and Chemical Reference Data. 24 (3): 1377–1381. Bibcode:1995JPCRD..24.1377R. doi:10.1063/1.555963. ISSN 0047-2689.
- ^ Lide 2003, 8—Concentrative Backdrop of Aqueous Solutions: Density, Refractive Index, Freezing Point Depression, and Viscosity.
- ^ Lide 2003, 6.186.
- ^ H2o in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Plant of Standards and Engineering science, Gaithersburg (Physician), http://webbook.nist.gov
- ^ Lide 2003, 9—Dipole Moments.
- ^ a b c Water in Linstrom, Peter J.; Mallard, William Chiliad. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Applied science, Gaithersburg (MD), http://webbook.nist.gov
- ^ GHS: PubChem 962
- ^ Greenwood & Earnshaw 1997, p. 620.
- ^ "Water, the Universal Solvent". U.S. Department of the Interior. usgs.gov (website). U.s.: USGS. October 22, 2019. Retrieved December 15, 2020.
- ^ Reece et al. 2013, p. 48.
- ^ a b c Weingärtner et al. 2016, p. 2.
- ^ Reece et al. 2013, p. 44.
- ^ "Autoprotolysis constant". IUPAC Compendium of Chemical Terminology. IUPAC. 2009. doi:10.1351/goldbook.A00532. ISBN978-0-9678550-nine-7.
- ^ Campbell, Williamson & Heyden 2006.
- ^ Smith, Jared D.; Christopher D. Cappa; Kevin R. Wilson; Ronald C. Cohen; Phillip L. Geissler; Richard J. Saykally (2005). "Unified description of temperature-dependent hydrogen bond rearrangements in liquid water" (PDF). Proc. Natl. Acad. Sci. USA. 102 (40): 14171–14174. Bibcode:2005PNAS..10214171S. doi:ten.1073/pnas.0506899102. PMC1242322. PMID 16179387.
- ^ Deguchi, Shigeru; Tsujii, Kaoru (2007-06-19). "Supercritical water: a fascinating medium for soft matter". Soft Matter. 3 (7): 797–803. Bibcode:2007SMat....3..797D. doi:10.1039/b611584e. ISSN 1744-6848. PMID 32900070.
- ^ Rhein, K.; Rintoul, South.R. (2013). "3: Observations: Ocean" (PDF). IPCC WGI AR5 (Written report). p. 257.
Ocean warming dominates the global energy change inventory. Warming of the ocean accounts for about 93% of the increase in the World's energy inventory between 1971 and 2010 (loftier confidence), with the warming of the upper (0 to 700 m) ocean bookkeeping for nearly 64% of the full. Melting ice (including Arctic body of water water ice, ice sheets, and glaciers) and warming of the continents and atmosphere account for the remainder of the alter in energy.
- ^ Lide 2003, Chapter 6: Properties of Ice and Supercooled H2o.
- ^ Lide 2003, 6. Backdrop of H2o and Steam as a Function of Temperature and Pressure.
- ^ "Prescript on weights and measures". April 7, 1795. Archived from the original on February 25, 2013. Retrieved Baronial iii, 2016.
Gramme, le poids absolu d'un book d'eau pure égal au cube de la centième partie du mètre, et à la température de la glace fondante.
- ^ a b c Greenwood & Earnshaw 1997, p. 625.
- ^ Shell, Scott M.; Debenedetti, Pablo G.; Panagiotopoulos, Athanassios Z. (2002). "Molecular structural club and anomalies in liquid silica" (PDF). Phys. Rev. Eastward. 66 (1): 011202. arXiv:cond-mat/0203383. Bibcode:2002PhRvE..66a1202S. doi:10.1103/PhysRevE.66.011202. PMID 12241346. S2CID 6109212. Archived from the original (PDF) on 2016-06-04. Retrieved 2009-07-07 .
- ^ Bullis, W. Murray (1990). "Affiliate 6". In O'Mara, William C.; Herring, Robert B.; Hunt, Lee P. (eds.). Handbook of semiconductor silicon technology. Park Ridge, New Bailiwick of jersey: Noyes Publications. p. 431. ISBN0-8155-1237-6 . Retrieved 2010-07-xi .
- ^ a b c d e Perlman, Howard. "Water Density". The USGS Water Science School . Retrieved 2016-06-03 .
- ^ a b Housecroft, Catherine Due east.; Sharpe, Alan G. (2005). Inorganic Chemistry (second ed.). Pearson Prentice-Hall. pp. 162–163. ISBN0130-39913-2.
- ^ Loerting, Thomas; Salzmann, Christoph; Kohl, Ingrid; Mayer, Erwin; Hallbrucker, Andreas (2001-01-01). "A second distinct structural "state" of high-density amorphous water ice at 77 K and i bar". Concrete Chemistry Chemical Physics. three (24): 5355–5357. Bibcode:2001PCCP....3.5355L. doi:x.1039/b108676f. ISSN 1463-9084.
- ^ Greenwood & Earnshaw 1997, p. 624.
- ^ Zumdahl & Zumdahl 2013, p. 493.
- ^ a b c "Can the ocean freeze?". National Body of water Service. National Oceanic and Atmospheric Administration. Retrieved 2016-06-09 .
- ^ Fine, R.A.; Millero, F.J. (1973). "Compressibility of water as a role of temperature and pressure". Journal of Chemical Physics. 59 (10): 5529. Bibcode:1973JChPh..59.5529F. doi:10.1063/one.1679903.
- ^ Neumeier, J.J. (2018). "Elastic Constants, Majority Modulus, and Compressibility of H2O Ice Ih for the Temperature Range 50 One thousand-273 G". Journal of Physical and Chemical Reference Data. 47 (iii): 033101. Bibcode:2018JPCRD..47c3101N. doi:10.1063/ane.5030640. S2CID 105357042.
- ^ "Base unit definitions: Kelvin". National Found of Standards and Technology. Retrieved 9 August 2018.
- ^ a b Weingärtner et al. 2016, p. v.
- ^ Proceedings of the 106th meeting (PDF). International Commission for Weights and Measures. Sèvres. 16–20 Oct 2017.
- ^ Schlüter, Oliver (2003-07-28). "Impact of High Pressure level — Low Temperature Processes on Cellular Materials Related to Foods" (PDF). Technischen Universität Berlin. Archived from the original (PDF) on 2008-03-09.
- ^ Tammann, Gustav H.J.A (1925). "The States Of Assemblage". Lawman And Company.
- ^ Lewis & Rice 1922.
- ^ Murphy, D. 1000. (2005). "Review of the vapour pressures of ice and supercooled h2o for atmospheric applications". Quarterly Journal of the Royal Meteorological Society. 131 (608): 1539–1565. Bibcode:2005QJRMS.131.1539M. doi:10.1256/qj.04.94. S2CID 122365938.
- ^ Debenedetti, P. One thousand.; Stanley, H. E. (2003). "Supercooled and Glassy Water" (PDF). Physics Today. 56 (6): forty–46. Bibcode:2003PhT....56f..40D. doi:10.1063/one.1595053.
- ^ Sharp 1988, p. 27.
- ^ "Revised Release on the Pressure forth the Melting and Sublimation Curves of Ordinary Water Substance" (PDF). IAPWS. September 2011. Retrieved 2013-02-19 .
- ^ C. South. Fuller "Defect Interactions in Semiconductors" Chapter five pp. 192-221 in "Semiconductors" Northward. B. Hannay Ed. Reinhold, New York 1959
- ^ a b Low-cal, Truman S.; Licht, Stuart; Bevilacqua, Anthony C.; Morash, Kenneth R. (2005-01-01). "The Fundamental Conductivity and Resistivity of Water". Electrochemical and Solid-State Letters. 8 (i): E16–E19. doi:x.1149/1.1836121. ISSN 1099-0062.
- ^ Crofts, A. (1996). "Lecture 12: Proton Conduction, Stoichiometry". Academy of Illinois at Urbana-Champaign. Retrieved 2009-12-06 .
- ^ Hoy, AR; Bunker, PR (1979). "A precise solution of the rotation angle Schrödinger equation for a triatomic molecule with application to the water molecule". Journal of Molecular Spectroscopy. 74 (1): 1–8. Bibcode:1979JMoSp..74....1H. doi:10.1016/0022-2852(79)90019-5.
- ^ Zumdahl & Zumdahl 2013, p. 393.
- ^ Campbell & Farrell 2007, pp. 37–38.
- ^ Campbell & Reece 2009, p. 47.
- ^ Chiavazzo, Eliodoro; Fasano, Matteo; Asinari, Pietro; Decuzzi, Paolo (2014). "Scaling behaviour for the water ship in nanoconfined geometries". Nature Communications. 5: 4565. Bibcode:2014NatCo...5.4565C. doi:ten.1038/ncomms4565. PMC3988813. PMID 24699509.
- ^ "Physical Forces Organizing Biomolecules" (PDF). Biophysical Gild. Archived from the original on August seven, 2007.
{{cite spider web}}: CS1 maint: unfit URL (link) - ^ Lide 2003, Surface Tension of Common Liquids.
- ^ a b c Reece et al. 2013, p. 46.
- ^ Zumdahl & Zumdahl 2013, pp. 458–459.
- ^ Greenwood & Earnshaw 1997, p. 627.
- ^ Zumdahl & Zumdahl 2013, p. 518.
- ^ Pugliano, N. (1992-11-01). "Vibration-Rotation-Tunneling Dynamics in Small H2o Clusters". Lawrence Berkeley Lab., CA (United States): 6. doi:10.2172/6642535. OSTI 6642535.
- ^ Richardson, Jeremy O.; Pérez, Cristóbal; Lobsiger, Simon; Reid, Adam A.; Temelso, Berhane; Shields, George C.; Kisiel, Zbigniew; Wales, David J.; Pate, Brooks H.; Althorpe, Stuart C. (2016-03-18). "Concerted hydrogen-bond breaking by quantum tunneling in the water hexamer prism". Science. 351 (6279): 1310–1313. Bibcode:2016Sci...351.1310R. doi:x.1126/science.aae0012. ISSN 0036-8075. PMID 26989250.
- ^ Kolesnikov, Alexander I. (2016-04-22). "Breakthrough Tunneling of Water in Beryl: A New Land of the Water Molecule". Concrete Review Letters. 116 (16): 167802. Bibcode:2016PhRvL.116p7802K. doi:10.1103/PhysRevLett.116.167802. PMID 27152824.
- ^ Pope; Fry (1996). "Absorption spectrum (380-700nm) of pure h2o. II. Integrating cavity measurements". Applied Optics. 36 (33): 8710–23. Bibcode:1997ApOpt..36.8710P. doi:ten.1364/ao.36.008710. PMID 18264420.
- ^ Brawl, Philip (2008). "Water—an enduring mystery". Nature. 452 (7185): 291–292. Bibcode:2008Natur.452..291B. doi:ten.1038/452291a. PMID 18354466. S2CID 4365814.
- ^ Gonick, Larry; Criddle, Craig (2005-05-03). "Chapter 3 Togetherness". The cartoon guide to chemistry (1st ed.). HarperResource. p. 59. ISBN9780060936778.
Water, H2O, is like. It has two electron pairs with cypher attached to them. They, too, must be taken into account. Molecules like NH3 and H2O are chosen bent.
- ^ Theodore L. Dark-brown; et al. (2015). "9.2 The Vsepr Model". Chemistry : the central science (thirteen ed.). p. 351. ISBN978-0-321-91041-7 . Retrieved 21 April 2019.
Notice that the bond angles decrease as the number of nonbonding electron pairs increases. A bonding pair of electrons is attracted by both nuclei of the bonded atoms, but a nonbonding pair is attracted primarily by but one nucleus. Because a nonbonding pair experiences less nuclear attraction, its electron domain is spread out more in space than is the electron domain for a bonding pair (Figure 9.7). Nonbonding electron pairs, therefore, have up more than space than bonding pairs; in essence, they deed every bit large and fatter balloons in our illustration of Effigy 9.five. As a event, electron domains for nonbonding electron pairs exert greater repulsive forces on side by side electron domains and tend to compress bond angles
- ^ Boyd 2000, p. 105.
- ^ Boyd 2000, p. 106.
- ^ "Guideline on the Use of Key Concrete Constants and Basic Constants of Water" (PDF). IAPWS. 2001.
- ^ Hardy, Edme H.; Zygar, Astrid; Zeidler, Manfred D.; Holz, Manfred; Sacher, Frank D. (2001). "Isotope outcome on the translational and rotational motion in liquid water and ammonia". J. Chem. Phys. 114 (7): 3174–3181. Bibcode:2001JChPh.114.3174H. doi:10.1063/ane.1340584.
- ^ Urey, Harold C.; et al. (fifteen Mar 1935). "Concerning the Taste of Heavy Water". Science. Vol. 81, no. 2098. New York: The Science Printing. p. 273. Bibcode:1935Sci....81..273U. doi:x.1126/scientific discipline.81.2098.273-a.
- ^ "Experimenter Drinks 'Heavy Water' at $5,000 a Quart". Pop Science Monthly. Vol. 126, no. 4. New York: Popular Scientific discipline Publishing. Apr 1935. p. 17. Retrieved seven Jan 2011.
- ^ Müller, Grover C. (June 1937). "Is 'Heavy H2o' the Fountain of Youth?". Popular Science Monthly. Vol. 130, no. vi. New York: Popular Science Publishing. pp. 22–23. Retrieved vii January 2011.
- ^ Miller, Inglis J., Jr.; Mooser, Gregory (Jul 1979). "Gustation Responses to Deuterium Oxide". Physiology & Behavior. 23 (one): 69–74. doi:10.1016/0031-9384(79)90124-0. PMID 515218. S2CID 39474797.
- ^ Weingärtner et al. 2016, p. 29.
- ^ Zumdahl & Zumdahl 2013, p. 659.
- ^ a b Zumdahl & Zumdahl 2013, p. 654.
- ^ Zumdahl & Zumdahl 2013, p. 984.
- ^ Zumdahl & Zumdahl 2013, p. 171.
- ^ "Hydrides". Chemwiki. UC Davis. 2 Oct 2013. Retrieved 2016-06-25 .
- ^ Zumdahl & Zumdahl 2013, pp. 932, 936.
- ^ Zumdahl & Zumdahl 2013, p. 338.
- ^ Zumdahl & Zumdahl 2013, p. 862.
- ^ Zumdahl & Zumdahl 2013, p. 981.
- ^ Charlot 2007, p. 275.
- ^ a b Zumdahl & Zumdahl 2013, p. 866.
- ^ a b Greenwood & Earnshaw 1997, p. 601.
- ^ "Enterprise and electrolysis..." Purple Society of Chemistry. Baronial 2003. Retrieved 2016-06-24 .
- ^ "Joseph Louis Gay-Lussac, French chemist (1778–1850)". 1902 Encyclopedia. Footnote 122-one. Retrieved 2016-05-26 .
- ^ Lewis, G. N.; MacDonald, R. T. (1933). "Concentration of H2 Isotope". The Journal of Chemic Physics. 1 (6): 341. Bibcode:1933JChPh...1..341L. doi:ten.1063/1.1749300.
- ^ a b Leigh, Favre & Metanomski 1998, p. 34.
- ^ IUPAC 2005, p. 85.
- ^ Leigh, Favre & Metanomski 1998, p. 99.
- ^ "Tetrahydropyran". Pubchem. National Institutes of Wellness. Retrieved 2016-07-31 .
- ^ Leigh, Favre & Metanomski 1998, pp. 27–28.
- ^ "Compound Summary for CID 22247451". Pubchem Chemical compound Database. National Eye for Biotechnology Information.
Bibliography [edit]
- Boyd, Claude E. (2000). "pH, Carbon Dioxide, and Alkalinity". Water Quality. Boston, Massachusetts: Springer. pp. 105–122. doi:x.1007/978-1-4615-4485-2_7. ISBN9781461544852.
- Campbell, Mary K.; Farrell, Shawn O. (2007). Biochemistry (6th ed.). Cengage Learning. ISBN978-0-495-39041-1.
- Campbell, Neil A.; Reece, Jane B. (2009). Biological science (eighth ed.). Pearson. ISBN978-0-8053-6844-iv.
- Campbell, Neil A.; Williamson, Brad; Heyden, Robin J. (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall. ISBN978-0-xiii-250882-7.
- Charlot, K. (2007). Qualitative Inorganic Analysis. Read Books. ISBN978-1-4067-4789-8.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemical science of the Elements (2nd ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- International Union of Pure and Applied Chemistry (2005-11-22). Classification of Inorganic Chemistry: IUPAC Recommendations 2005 (PDF). Purple Society of Chemistry. ISBN978-0-85404-438-2 . Retrieved 2016-07-31 .
- Leigh, Yard. J.; Favre, H. A; Metanomski, W. V. (1998). Principles of chemical classification: a guide to IUPAC recommendations (PDF). Oxford: Blackwell Science. ISBN978-0-86542-685-half-dozen. OCLC 37341352. Archived from the original (PDF) on 2011-07-26.
- Lewis, William C.M.; Rice, James (1922). A System of Physical Chemistry. Longmans, Green and Co.
- Lide, David R. (2003-06-19). CRC Handbook of Chemistry and Physics, 84th Edition. CRC Handbook. CRC Printing. ISBN9780849304842.
- Reece, Jane B.; Urry, Lisa A.; Cain, Michael Fifty.; Wasserman, Steven A.; Minorsky, Peter Five.; Jackson, Robert B. (2013-11-10). Campbell Biology (10th ed.). Boston, Mass.: Pearson. ISBN9780321775658.
- Riddick, John (1970). Organic Solvents Physical Properties and Methods of Purification . Techniques of Chemical science. Wiley-Interscience. ISBN978-0471927266.
- Sharp, Robert Phillip (1988-11-25). Living Ice: Understanding Glaciers and Glaciation . Cambridge University Press. p. 27. ISBN978-0-521-33009-i.
- Weingärtner, Hermann; Teermann, Ilka; Borchers, Ulrich; Balsaa, Peter; Lutze, Holger Five.; Schmidt, Torsten C.; Franck, Ernst Ulrich; Wiegand, Gabriele; Dahmen, Nicolaus; Schwedt, Georg; Frimmel, Fritz H.; Gordalla, Birgit C. (2016). "Water, 1. Backdrop, Analysis, and Hydrological Cycle". Ullmann'south Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA. doi:10.1002/14356007.a28_001.pub3. ISBN9783527306732.
- Zumdahl, Steven S.; Zumdahl, Susan A. (2013). Chemical science (9th ed.). Cengage Learning. ISBN978-1-13-361109-7.
Further reading [edit]
- Ben-Naim, A. (2011), Molecular Theory of Water and Aqueous Solutions, World Scientific
External links [edit]
- "Water Properties and Measurements". U.s.a. Geological Survey. May 2, 2016. Retrieved August 31, 2016.
- Release on the IAPWS Conception 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use (simpler formulation)
- Online calculator using the IAPWS Supplementary Release on Properties of Liquid Water at 0.1 MPa, September 2008
- Chaplin, Martin (2019). "Structure and Properties of H2o in its Various States". Encyclopedia of Water. Wiley Online Library 2019. pp. 1–xix. doi:x.1002/9781119300762.wsts0002. ISBN9781119300755. S2CID 213738895.
- Calculation of vapor pressure level, liquid density, dynamic liquid viscosity, and surface tension of water
- Water Density Calculator
- Why does ice float in my drink?, NASA
Source: https://en.wikipedia.org/wiki/Properties_of_water
Posted by: smithwiton1980.blogspot.com



![K_{{{\rm {w}}}}=[{{\rm {{H_{3}O^{+}}}}}][{{\rm {{OH^{-}}}}}]](https://wikimedia.org/api/rest_v1/media/math/render/svg/86dca39006c4f875cacc14395c7ff6e38a09d990)

![{\displaystyle K_{\rm {eq}}\approx K_{\rm {w}}=[{\rm {H_{3}O^{+}}}][{\rm {OH^{-}}}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8c479a6b2710d07dd3952fcc072550c0e8537e70)
0 Response to "What Animal Uses The Proteites Of Density To Function"
Post a Comment